Vitamin C Supplement Label
Dietary Supplement Labeling Guide: Chapter IV. Nutrition Labeling
April 2005
Contains Nonbinding Recommendations
Return to Table of Contents
Questions
General
- What is the nutrition label for a dietary supplement called?
- How does "Supplement Facts" differ from "Nutrition Facts"?
- What information must I list in the "Supplement Facts" panel?
Serving Size
- What is the serving size for a dietary supplement?
- May I use flexibility in the wording for "Serving Size"?
Nutrient Declaration
- What nutrients am I required to list in the "Supplement Facts" panel?
- Must I declare vitamins and minerals (other than vitamin A, vitamin C, calcium, and iron) listed in 21 CFR 101.9(c)(8)(iv) and (c)(9)?
- Am I required to list any other nutrients if I make a claim about them?
- May I declare dietary ingredients not having Daily Values (i.e., RDIs or DRVs)?
- If I use a magnesium salt as a binder, where must I declare it?
- Must I declare vitamin E when it occurs naturally in my product and I make no claim for it?
- May I declare protein on the label if my product contains only individual amino acids?
- Must I list the dietary ingredients in my products in a specified order?
- May I use synonyms for my dietary ingredients?
Amounts
- If the calcium carbonate in my product supplies calcium, should I list the weight of the entire salt or just of the calcium?
- May I list the amount of my dietary ingredient in a separate column?
- When I use a separate column for amounts, can the heading "Amount Per Serving" be placed over the column of amounts?
- May I use language other than the term "Amount Per Serving"?
- May I present information on the "Amount Per Unit" basis?
- May I present information on more than one serving?
- Am I required to use the units of measurement specified for use in the "Nutrition Facts" panel?
Percent of Daily Value (%DV)
- What is the % DV?
- Do I need to list the % DV on my label?
- How do I calculate the % DV?
- What rounding rules must I use for expressing the % DV?
- What if the amount of a dietary ingredient present in my product is high enough to declare, but so low that the % DV rounds to zero?
- Can I show more than one column for % DVs?
Other Dietary Ingredients
- What are "other dietary ingredients"?
- Where must I list "other dietary ingredients"?
- How must I list "other dietary ingredients"?
- How must I list liquid extracts?
- How must I list dried extracts?
- May I list constituents of a dietary ingredient?
- How must I list proprietary blends?
Format
- How must I display the "Supplement Facts" panel?
- How must I present the information in the "Supplement Facts" panel?
- What are the type size requirements for the "Supplement Facts" panel?
- Must I use hairlines in the "Supplement Facts" panel?
- How closely must I follow the "Examples of Graphic Enhancements Used by the FDA" in Appendix B to Part 101?
- How do I provide nutrition labeling when my product contains two or more packets of supplements (e.g., a packet of capsules for the morning and a different packet for the evening)?
Compliance
- What kind of samples will FDA collect to determine compliance with 21 CFR 101.36?
- What if it is not technically feasible for me to comply with the nutrition labeling requirements?
- Must dietary ingredients that I have added to my products be present at 100% of the amount that I declare?
Exemptions
- What are the circumstances in which my dietary supplement products would be exempt from the nutrition labeling requirements?
Special Labeling Provisions
- What are small packages?
- What is the telephone provision for small packages?
- What is the minimum type size that I may use for small packages?
- May I use a tabular or linear format for the "Supplement Facts" panel on a small package?
- What are intermediate-sized packages?
- What is the minimum type size for intermediate-sized packages?
- May I use a tabular or linear format for the "Supplement Facts" panel on an intermediate-sized panel?
- May I abbreviate on the labels of intermediate-sized packages?
- Must I always use hairlines on the labels of intermediate-sized packages?
- Are there special requirements that I must follow for the labeling of dietary supplements for children?
- Must I include a footnote comparing a 2,000 calorie diet to a 2,500 calorie diet in the "Supplement Facts" of my product?
- May I locate the "Supplement Facts" panel on other than the information panel?
- May I omit the "Supplement Facts" panel on individual unit containers in multi-unit retail packs?
- How do I provide the "Supplement Facts" panel if my dietary supplements are sold from bulk containers?
- Does FDA have sample labels for dietary supplements?
Answers
General
- What is the nutrition label for a dietary supplement called?
The nutrition label for a dietary supplement is called a "Supplement Facts" panel (see sample labels at the end of this chapter).
21 CFR 101.36(b)(1)(i)
- How does "Supplement Facts" differ from "nutrition facts?"
The major differences between "Supplement Facts" panel and "Nutrition Facts" panel are as follows:
- You must list dietary ingredients without RDIs or DRVs in the "Supplement Facts" panel for dietary supplements. You are not permitted to list these ingredients in the "Nutrition Facts" panel for foods.
- You may list the source of a dietary ingredient in the "Supplement Facts" panel for dietary supplements. You cannot list the source of a dietary ingredient in the "Nutrition Facts" panel for foods.
- You are not required to list the source of a dietary ingredient in the ingredient statement for dietary supplements if it is listed in the "Supplement Facts" panel.
- You must include the part of the plant from which a dietary ingredient is derived in the "Supplement Facts" panel for dietary supplements. You are not permitted to list the part of a plant in the "Nutrition Facts" panel for foods.
- You are not permitted to list "zero" amounts of nutrients in the "Supplement Facts" panel for dietary supplements. You are required to list "zero" amounts of nutrients in the "Nutrition Facts" panel for food.
21 CFR 101.36(b)(3) and (b)(2)(i), 21 CFR 101.4(h), 21 CFR 101.36(d) and (d)(1), and 21 CFR 101.9
- What information must I list in the "Supplement Facts" panel?
You must list the names and quantities of dietary ingredients present in your product, the "Serving Size" and the "Servings Per Container." However, the listing of "Servings Per Container" is not required when it is the same information as in the net quantity of contents statement. For example, when the net quantity of contents statement is 100 tablets and the "Serving Size" is one tablet, the "Serving Per Container" also would be 100 tablets and would not need to be listed.
21 CFR 101.36(b)
Serving Size
- What is the serving size for a dietary supplement?
One serving of a dietary supplement equals the maximum amount recommended, as appropriate, on the label for consumption per eating occasion, or in the absence of recommendations, 1 unit (e.g., tablet, capsule, packet, teaspoonful, etc). For example, if the directions on your label say to take 1-3 tablets with breakfast, the serving size would be 3 tablets.
21 CFR 101.12(b) Table 2 in the Miscellaneous Category
- May I use flexibility in the wording for "Serving Size?"
No. You must use the term "Serving Size."
21 CFR 101.36(b)(1)
Nutrient Declaration
- What nutrients am I required to list in the "Supplement Facts" panel?
Total calories, calories from fat, total fat, saturated fat, cholesterol, sodium, total carbohydrate, dietary fiber, sugars, protein, vitamin A, vitamin C, calcium, and iron must be listed when they are present in measurable amounts. A measurable amount is an amount that exceeds the amount that can be declared as "zero" in the nutrition label of conventional foods, as specified in 21 CFR 101.9(c). If present in a measurable amount, trans fat must be listed on a separate line underneath the listing of saturated fat by January 1, 2006.
Calories from saturated fat and the amount of polyunsaturated fat, monounsaturated fat, soluble fiber, insoluble fiber, sugar alcohol, and other carbohydrate may be declared, but they must be declared when a claim is made about them.
21 CFR 101.36(b)(2)(i) (see 68 FR 41434 at 41505, July 11, 3003)
- Must I declare vitamins and minerals (other than vitamin A, vitamin C, calcium, and iron) listed in 21 CFR 101. 9(c)(8)(iv) and (c)(9)?
No. You are only required to declare them when they are added to the product for purposes of supplementation, or if you make a claim about them.
21 CFR 101.36(b)(2)(i)
- Am I required to list any other nutrients if I make a claim about them?
Yes. When you make a claim about calories from saturated fat, insoluble fiber, polyunsaturated fat, sugar alcohol, monounsaturated fat, other carbohydrate, and soluble fiber, you must list that nutrient.
21 CFR 101.36(b)(2)(i)
- May I declare dietary ingredients not having Daily Values (i.e., RDIs or DRVs)?
Yes. Dietary ingredients for which no daily values have been established must be listed by their common or usual names when they are present in a dietary supplement. They must be identified as having no Daily Values by use of a symbol in the column for "% Daily Value" that refers to the footnote "Daily Value Not Established."
21 CFR 101.36(b)(2)(iii)(F) and (b)(3)
- If I use a magnesium salt as a binder, where must I declare it?
You must list the specific magnesium salt in the ingredient statement below the "Supplement Facts" panel, not in the "Nutrition Facts" panel. Ingredients in dietary supplements that are not dietary ingredients, such as binders, excipients, fillers, must be included in the ingredient statement.
21 CFR 101.4(g)
- Must I declare vitamin E when it occurs naturally in my product and I make no claim for it?
No. Because Vitamin E is not one of the 14 mandatory dietary ingredients, it does not need to be declared when it occurs naturally.
21 CFR 101.36(b)(2)(i)
- May I declare protein on the label if my product contains only individual amino acids?
No. You may not declare protein on your products that contain only amino acids.
21 CFR 101.36(b)(2)(i)
- Must I list the dietary ingredients in my products in a specified order?
Yes. You must list the dietary ingredients that have Daily Values in the same order as for the labels of conventional foods, except that vitamins, minerals and electrolytes are grouped together. This results in the following order for vitamins and minerals: Vitamin A, vitamin C, vitamin D, vitamin E, vitamin K, thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, biotin, pantothenic acid, calcium, iron, phosphorus, iodine, magnesium, zinc, selenium, copper, manganese, chromium, molybdenum, chloride, sodium, and potassium.
21 CFR 101.36(b)(2)(i)(B)
- May I use synonyms for my dietary ingredients?
Yes. You may use the following synonyms in parentheses after your dietary ingredients: Vitamin C (ascorbic acid), thiamin (vitamin B1), riboflavin (vitamin B2), folate (folacin or folic acid), and calories (energy). Alternatively, you may list "folic acid" or "folacin" without parentheses in place of "folate." You may also express energy content parenthetically in kilojoules immediately following the caloric content.
21 CFR 101.36(b)(2)(i)(B)(2)
Amounts
- If the calcium carbonate in my product supplies calcium , should I list the weight of the entire salt or just of the calcium?
You must list the weight of calcium, rather than the weight of the calcium carbonate, the source ingredient, in the "Supplement Facts" panel.
21 CFR 101.36(b)(2)(ii)
- May I list the amount of my dietary ingredient in a separate column?
Yes. You may place the amount of your dietary ingredient in a separate column or immediately following the name of your dietary ingredient.
21 CFR 101.36(b)(2)(ii)
- When I use a separate column for amounts, can the heading "Amount per Serving" be placed over the column of amounts?
Yes.
21 CFR 101.36(b)(2)(i)(A)
- May I use language other than the term "Amount Per Serving?"
Yes. Language consistent with the declaration of the serving size, such as "Each Tablet Contains" or "Amount Per 2 Tablets" may be used in place of the heading "Amount Per Serving." You may also use terms, such as capsule, packet, or teaspoonful.
21 CFR 101.36(b)(2)(i)(A)
- May I present information on the "Amount Per Unit" basis?
Yes. You may declare information on a "per unit" basis in addition to the required "per serving" basis.
21 CFR 101.36(b)(2)(iv)
- May I present information on more than one serving?
Yes. You may use additional columns when you have a product with different servings, such as one tablet in the morning and two at night. You must label the columns appropriately, e.g., "Amount per 1 Tablet" and "Amount per 2 Tablets."
21 CFR 101.36(b)(2)(i)(A)
- Am I required to use the units of measurement specified for use in the "Nutrition Facts" panel?
Yes. For example, the amount of fat would be listed in terms of grams in both the "Nutrition Facts" and "Supplement Facts" panels. However, units of measurement for amounts of vitamins and minerals are not specified for use in the "Nutrition Facts" panel because they must be listed by % Daily Value, not by weight. You should use the units of measurement given in 21 CFR 101.9(c)(8)(iv) for the Daily Values of vitamins and minerals when listing these nutrients in "Supplement Facts" (e.g., the amount of vitamin C must be listed in terms of milligrams because its Daily Value is stated in milligrams).
21 CFR 101.36(b)(2)(ii)(B) and 101.9(c)
Percent Of Daily Value (% DV)
- What is the % DV?
The % DV is the percent of the Daily Value (i.e., Reference Daily Intakes or Daily Reference Value) of a dietary ingredient that is in a serving of the product.
21 CFR 101.36(b)(2)(ii)(B) and 21 CFR 101.9(c)(8) and (9)
- Do I need to list the % DV on my label?
The % DV must be declared for all dietary ingredients for which FDA has established Daily Values, except that 1) the percent for protein may be omitted, and 2) on the labels of dietary supplements to be used by infants, children less than 4 years of age, or pregnant or lactating women, you must not list any percent for total fat, saturated fat, cholesterol, total carbohydrate, dietary fiber, vitamin K, selenium, manganese, chromium, molybdenum, chloride, sodium, or potassium. See Appendix B for the daily values to be used for adults and children 4 or more years of age and Appendix C for the daily values to be used for infants, children less than 4 years of age, or pregnant or lactating women.
21 CFR 101.36(b)(2)(iii)
- How do I calculate the % DV?
You calculate the % DV by dividing the quantitative amount by weight by the established Daily Value for the specified dietary ingredient and multiplying by 100 (except that the % DV for protein must be calculated in accordance with 21 CFR 101.9(c)(7)(iii)). In this calculation, you must use as the quantitative amount the unrounded amount, except that for total fat, saturated fat, cholesterol, sodium, potassium, total carbohydrate, and dietary fiber, you may use the quantitative amount by weight declared on the label (i.e., the rounded amount). For example, the % DV for 60 mg of vitamin C is 100 (60 mg divided by the Daily Value for vitamin C, multiplied by 100).
21 CFR 101.36(b)(2)(iii)(B) and 21 CFR 101.9(c)(7)(iii)
- What rounding rules must I use for expressing the % DV?
You must express the percentages to the nearest whole percent, except that "Less than 1 %" or "< 1 %" must be used when the amount present is big enough to be listed, but so small that the % DV when rounded to the nearest percent is zero. For example, a product containing 1 gram of total carbohydrate would list the % DV as "Less than 1 %" or "< 1 %."
21 CFR 101.36(b)(2)(iii)(C)
- What if the amount of a dietary ingredient present in my product is high enough to declare, but so low that the % DV rounds to zero?
You must declare "Less than 1%" or "< 1%" because your label might confuse consumers if you declare 5 mg and list 0% DV. For example, if a product contains 5 mg of potassium, the % DV calculates to 0.14 percent (5 mg divided by 3,500 mg), which you would round to zero. In this case, you would declare "Less than 1%" or "< 1%" for the % DV.
Note: This does not pertain to dietary ingredients having RDIs because they may not be listed when present at less than 2 percent of the RDI.
21 CFR 101.36(b)(2)(iii)(C) and 101.36(b)(2)(i)
- Can I show more than one column for % DVs?
Yes. You may show more than one column. FDA has established four sets of Daily Values for many nutrients. Appendix B shows the Daily Values to be used for adults and children 4 or more years of age and Appendix C has the Daily Values to be used for children under 4 years of age, for infants, and for pregnant and lactating women. When you show more than one column, you must clearly identify each column (e.g., % Daily Value for Children Under 4 Years of Age).
21 CFR 101.36(b)(2)(iii)(E) and (e)(10)(ii)
Other Dietary Ingredients
- What are "other dietary ingredients?"
"Other dietary ingredients" are those dietary ingredients that do not have Daily Values (i.e. RDIs or DRVs) such as phosphatidylserine.
21 CFR 101.36(b)(3)(i)
- Where must I list "other dietary ingredients?"
You must list "other dietary ingredients" in the "Supplement Facts" panel following the listing of dietary ingredients having Daily Values.
21 CFR 101.36(b)(3)(i)
- How must I list "other dietary ingredients?"
You must list "other dietary ingredients" by common or usual name in a column or linear display. FDA has not specified an order that you must follow. You must list the quantitative amount by weight per serving immediately following the name of the dietary ingredient or in a separate column. You must place a symbol in the column for "% Daily Value" that refers to the footnote "Daily Value Not Established," except that the symbol must follow the weight when you do not use the column format.
21 CFR 101.36(b)(3)
- How must I list liquid extracts?
You must list liquid extracts using the volume or weight of the total extract and the condition of the starting material prior to extraction when it was fresh. You may include information on the concentration of the dietary ingredient and the solvent used, e.g., "fresh dandelion root extract, x (y:z) in 70% ethanol," where "x" is the number of mL or mg of the entire extract, "y" is the weight of the starting material, and "z" is the volume (mL) of solvent. You must identify the solvent in either the nutrition label or ingredient list.
21 CFR 101.36(b)(3)(ii)(B)
- How must I list dried extracts?
For dietary ingredients that are extracts from which the solvent has been removed, you must list the weights of the dried extracts.
21 CFR 101.36(b)(3)(ii)(C)
- May I list constituents of a dietary ingredient?
Yes. You may list constituents of a dietary ingredient indented under the dietary ingredient and followed by their quantitative amounts by weight per serving. You may declare the constituents in a column or in a linear display.
21 CFR 101.36(b)(3)(iii)
- How must I list proprietary blends?
You must identify proprietary blends by use of the term "Proprietary Blend" or an appropriately descriptive term or fanciful name. On the same line, you must list the total weight of all "other dietary ingredients" contained in the blend. Indented underneath the name of the blend, you must list the "other dietary ingredients" in the blend, either in a column or linear fashion, in descending order of predominance by weight. These ingredients should be followed by a symbol referring to the footnote "Daily Value Not Established." Dietary ingredients having RDIs or DRVs must be listed separately and the individual weights declared.
21 CFR 101.36(b)(2) and (c)
Format
- How must I display the "Supplement Facts" panel?
The "Supplement Facts" nutrition information (referred to as a panel) must be enclosed in a box by using hairlines. The title, "Supplement Facts," must be larger than all other print in the panel and, unless impractical, must be set full width of the panel. The title and all headings must be bolded to distinguish them from other information.
21 CFR 101.36(e)
- How must I present the information in the "Supplement Facts" panel?
You must present all information using the following:
- A single easy-to-read type style;
- All black or one color type, printed on a white or neutral contrasting background, whenever practical;
- Upper- and lowercase letters, except that you may use all uppercase lettering on small packages (i.e., packages having a total surface area available to bear labeling of less than 12 square inches);
- At least one point leading (i.e., space between lines of text); and
- Letters that do not touch.
21 CFR 101.36(e)
- What are the type size requirements for the "Supplement Facts" panel?
Except as provided for small and intermediate-sized packages, you must set information other than the title, headings, and footnotes in uniform type size no smaller than 8 point. You also must use a type size larger than all other print size in the nutrition label for the title "Supplement Facts." You may set the column headings and footnotes in type no smaller than 6 point type. See the section on "Special Labeling Provisions" for the exceptions for small and intermediate-sized packages.
21 CFR 101.36(e)
- Must I use hairlines in the Supplement Facts panel?
Except for small and intermediate-sized packages, you must use a hairline rule that is centered between the lines of text to separate each dietary ingredient from the dietary ingredient above and beneath it. FDA has provided an exception for certain packages with space constraints. See the section on "Special Labeling Provisions" for the exceptions for small and intermediate-sized packages.
21 CFR 101.36(e)
- How closely must I follow the "Examples of graphic enhancements used by the FDA" in appendix B to Part 101?
You are not required to follow Appendix B to Part 101. Appendix B and its specifications are a model, which FDA has suggested in the interest of uniformity of presentation. For example, 21 CFR 101.36(e)(3)(i) requires the use of an "easy-to-read" type style, not specifically Helvetica type, as suggested in Appendix B.
21 CFR 101.36(e)(9)
- How do I provide nutrition labeling when my product contains two or more packets of supplements (e.g., a packet of capsules for the morning and a different packet for the evening)?
You may present the information for each packet (e.g., a packet of capsules for the morning and a different packet for the evening) in an individual nutrition label or you may use an aggregate nutrition label. For two packets, this would consist of five columns. List all of the dietary ingredients in the first column. List the amounts and percents of the morning packet in the second and third columns and similar information for the evening packet in the fourth and fifth columns (see the illustration of aggregate nutrition labeling in 21 CFR 101.36(e)(10)(iii)).
21 CFR 101.36(e)(8)
Compliance
- What kind of samples will FDA collect to determine compliance with 21 CFR 101.36?
FDA will collect a composite of 12 subsamples (consumer packages) or 10 percent of the number of packages in the same inspection lot, whichever is smaller. FDA will randomly select these packages.
21 CFR 101.36(f)(1)
- What if it is not technically feasible for me to comply with the nutrition labeling requirements?
FDA may permit you to use an alternative means of compliance or additional exemptions in accordance with 21 CFR 101.9(g)(9). If your firm needs such special allowances, you must make your request in writing to the Office of Nutritional Products, Labeling, and Dietary Supplements (HFS-800), Food and Drug Administration, 5100 Paint Branch Parkway, College Park, Maryland 20740-3835.
21 CFR 101.36(f)(2)
- Must dietary ingredients that I have added to my products be present at 100% of the amount that I declare?
For dietary ingredients that are specifically added, your product must contain 100% of the volume or weight that you have declared on the label, with the exception of a deviation that is attributable to the analytical method. Products that contain less than this amount of such a dietary ingredient would be misbranded and in violation of the law. Dietary ingredients that are naturally-occurring must be present at 80% of the declared value. For example, if you add vitamin C that was isolated from a natural source or made synthetically to your dietary supplement product, it would be subject to the 100% rule. However, if you added rose hips to your product, the vitamin C in the rose hips is naturally-occurring and must be present at least 80% of the declared value.
21 CFR 101.9(g)(3) and (g)(4)
Exemptions
- What are the circumstances in which my dietary supplement products would be exempt from the nutrition labeling requirements?
Your dietary supplement product is not required to have a "Supplement Facts" panel if:
- Your firm is a small business that has not more than $50,000 gross sales made or business done in sales of food to consumers or not more than $500,000 per year from total sales in accordance with 21 CFR 101.36(h)(1);
- You sell less than 100,000 units of the product annually, your firm has fewer than 100 full-time equivalent employees in accordance with 21 CFR 101.36(h)(2) and you file an annual notification with FDA as specified in 21 CFR 101.9(j)(18)(iv); or
- You ship the product in bulk form, do not distribute it to consumers in such form, and you supply it for use in the manufacture of other dietary supplements in accordance with 21 CFR 101.36(h)(3).
The two exemptions for small businesses and low-volume products (a. and b. above) are available to you only if your products' labels bear no claims or other nutrition information.
21 CFR 101.36(h)(1) - (3)
Special Labeling Provisions
- What are small packages?
Small packages are those packages having less than 12 square inches of total surface area available to bear labeling.
21 CFR 101.36(i)(2) and 21 CFR 101.9(j)(13)
- What is the telephone provision for small packages?
In lieu of a "Supplement Facts" panel, you may print labels for small packages with a telephone number or address that consumers can use to obtain nutrition information. You may use a telephone number or an address in place of the "Supplement Facts" panel only if you place no claims or other nutrition information on the product label.
21 CFR 101.36(i)(2) and 21 CFR 101.9(j)(13)(i)
- What is the minimum type size that I may use for small packages?
You may use a type size no smaller than 4.5 point for the "Supplement Facts" panel on the labels of small packages.
21 CFR 101.36(i)(2)(i)
- May I use a tabular or linear format for the "Supplement Facts" panel on a small package?
Yes. You may use a tabular format on small packages. You also may present "Supplement Facts" information in a linear (i.e., string) fashion if the label will not accommodate the "Supplement Facts" panel in a tabular format. (See 21 CFR 101.9(j)(13)(ii)(A)(1) for an illustration of a tabular display and 21 CFR 101.9(j)(13)(ii)(A)(2) for an illustration of a linear display.)
21 CFR 101.36(i)(2) and 21 CFR 101.9(j)(13)(ii)(A)
- What are intermediate-sized packages?
Intermediate-sized packages are those packages having from 12 to 40 square inches of total surface area available to bear labeling.
21 CFR 101.36(i)(2)(ii)
- What is the minimum type size for intermediate-sized packages?
The "Supplement Facts" panel on the labels of intermediate-sized packages must use type size no smaller than 6 point, except that type no smaller than 4.5 point may be used on packages that have 20 to 40 square inches that list more than 16 dietary ingredients. Also, 4.5 point type may be used on packages with less than 20 square inches that list more than 8 dietary ingredients.
Furthermore, the type size used in the "Supplement Facts" panel on an inner container may be as small as needed to accommodate all required information if the "Supplement Facts" on the outer container meets these type size requirements.
21 CFR 101.36(i)(2)(ii) and (i)(2)(iv)
- May I use a tabular or linear format for the "Supplement Facts" panel on an intermediate-sized package?
You may use a tabular format on an intermediate-sized package if the package shape or size cannot accommodate vertical columns. You may use a linear format if the label will not accommodate a tabular format. (See 21 CFR 101.9(j)(13)(ii)(A)(1) for an illustration of a tabular display and 21 CFR 101.9(j)(13)(ii)(A)(2) for an illustration of a linear display).
21 CFR 101.36(i)(2) and 21 CFR 101.9(j)(13)(ii)(A)
- May I abbreviate on the labels of intermediate- sized packages?
You may use the abbreviations in 21 CFR 101.9(j)(13)(ii)(B) in the "Supplement Facts" panel for small and intermediate-sized packages, e.g, "Serv size" for "Serving Size" and "Servings" for "Servings Per Container."
21 CFR 101.9(j)(13)(ii)(B)
- Must I always use hairlines on the labels of intermediate-sized packages?
No. You may use a row of dots connecting the columns containing the name of each dietary ingredient and the quantitative amount (by weight and as a percent of Daily Value) in the "Supplement Facts" panel on a small or an intermediate-sized package if you use the minimum type size and there is not sufficient space for you to use hairlines.
21 CFR 101.36(i)(2)(v)
- Are there special requirements that I must follow for the labeling of dietary supplements for children?
Yes. On products for children less than 2 years of age, other than infant formula, you must not declare calories from fat, calories from saturated fat, saturated fat, polyunsaturated fat, monounsaturated fat, and cholesterol. Also, on products for children less than 4 years of age, you may not include % DVs for total fat, saturated fat, cholesterol, total carbohydrate, dietary fiber, vitamin K, selenium, manganese, chromium, molybdenum, chloride, sodium, or potassium.
21 CFR 101.36(b)(2)(iii) and (i)(1)
- Must I include a footnote comparing a 2,000 calorie diet to a 2,500 calorie diet in the "Supplement Facts" panel of my product?
No. You are not required to place the footnote on dietary supplements that is required by 21 CFR 101.9(d)(9) on conventional foods. However, you are required to include the footnote "Percent Daily Values are based on a 2,000 calorie diet" when you declare total fat, saturated fat, total carbohydrate, dietary fiber, or protein.
21 CFR 101.36(b)(2)(iii)(D)
- May I locate the "Supplement Facts" panel on other than the information panel?
Yes. If there is insufficient space for the "Supplement Facts" panel on the information panel or the principal display panel, you may locate it on other panels that can readily be seen by consumers in accordance with 21 CFR 101.9(j)(17).
21 CFR 101.36(i)(2)(iii) and (i)(5) and 21 CFR 101.9(j)(17)
- May I omit the "Supplement Facts" panel on individual unit containers in multi-unit retail packs?
Yes. You may omit the "Supplement Facts" panel on individual units if nutrition information is fully provided on the outer package of the multi-unit pack and the unit containers are securely enclosed and are not intended to be separated for retail sale. You must label each individual unit with the statement "This Unit Not Labeled For Retail Sale" in accordance with 21 CFR 101.9(j)(15).
21 CFR 101.36(i)(3) and 21 CFR 101.9(j)(15)
- How do I provide the "Supplement Facts" panel if my dietary supplements are sold from bulk containers?
The retailer must display a "Supplement Facts" panel clearly at the point of purchase (e.g. on a counter card, sign, tag affixed to the product, or some other appropriate device). Alternatively, the required information may be placed in a booklet, looseleaf binder, or some other appropriate format that is available at the point of purchase.
21 CFR 101.36(i)(4), 21 CFR 101.9(a)(2) and (j)(16)
- Does FDA have sample labels for dietary supplements?
Yes. See sample labels below.
-
Sample Labels
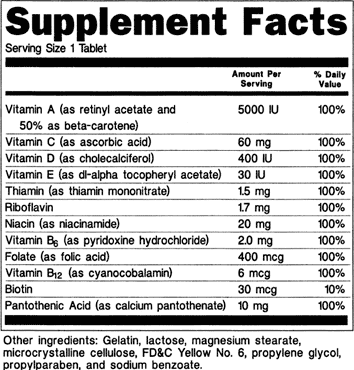
A) Dietary supplement containing multiple vitamins (see 21 CFR 101.36(e)(10)(i)):
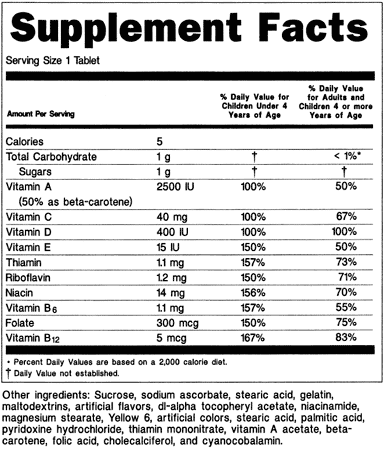
B) Dietary supplement containing multiple vitamins for children and adults (see 21 CFR 101.36(e)(10)(ii)):
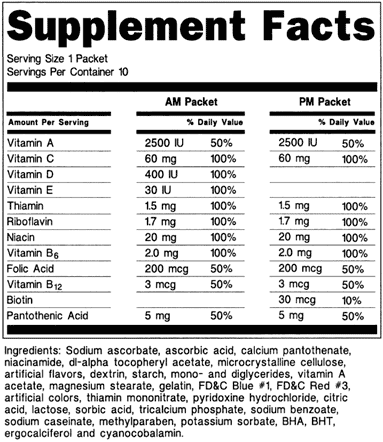
- C) Multiple vitamins in packets (see 21 CFR 101.36(e)(10)(iii)):
-
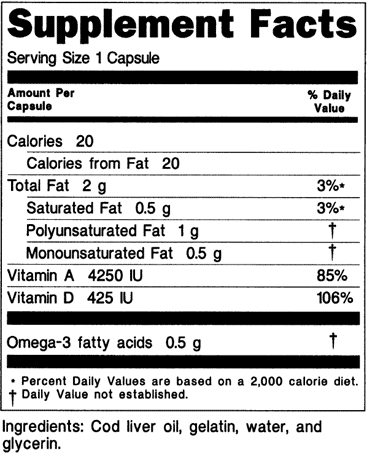
D) Dietary supplement containing dietary ingredients with and without RDIs and DRVs (see 21 CFR 101.36(e)(10)(iv):
Source: https://www.fda.gov/food/dietary-supplements-guidance-documents-regulatory-information/dietary-supplement-labeling-guide-chapter-iv-nutrition-labeling







Tidak ada komentar:
Posting Komentar